Customizing Mechanical Test Methods for Medical Device R&D
Free, 45-Minute Webinar
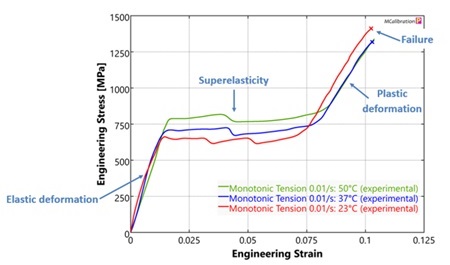
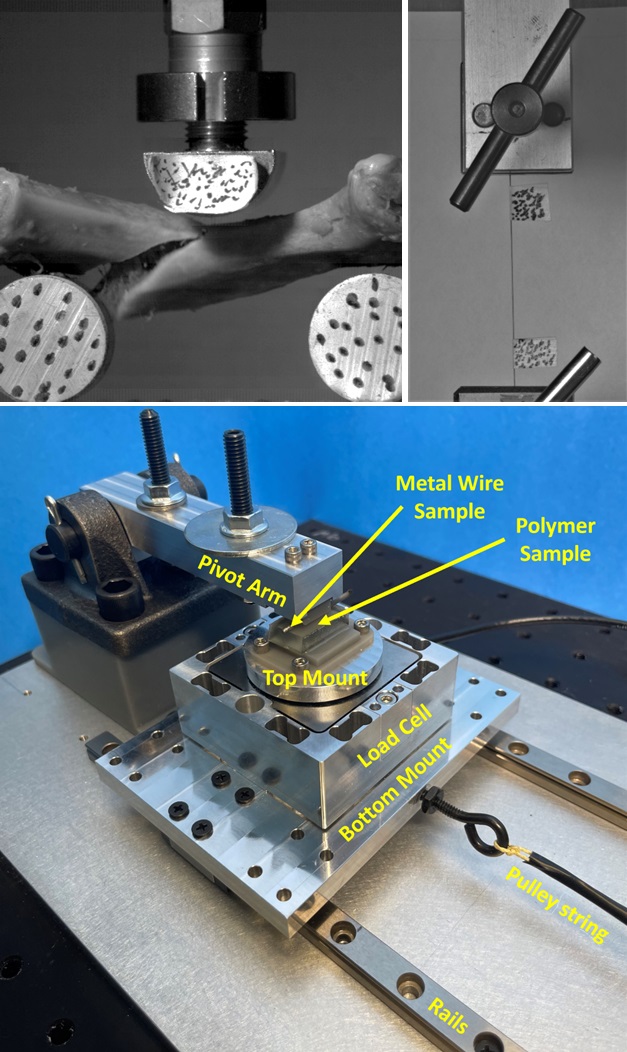
Medical devices are composed of many different materials with a wide range of mechanical properties. Quantifying these properties and how they are affected by factors such as temperature, chemical environment, loading rate, and manufacturing history is critical to ensure device performance. Evaluating these properties is also often necessary to calibrate material models for finite element analysis (FEA). It is often the case that standard test methods are insufficient due to part geometry, part size, and/or manufacturing method. In these cases, custom test methods are required.
This webinar will highlight some challenges associated with mechanical testing in the medical device space and share experimental approaches for addressing those challenges. We will share strategies for testing very small specimens, for testing synthetic and biological materials at impact strain rates, and for running tests to enable accurate calibration of material models for finite element analysis.
Details
Course Instructor
Nicole Mattson is an Engineer at Veryst Engineering. Her consulting focuses on standard and custom material testing, CAD design development and modification, and solid mechanics simulation in Abaqus.
With degrees in biomedical engineering, Nicole's experience has focused on skeletal biomechanics, with particular emphasis on the analysis of lower extremity bone quality following the use of an external orthopedic device. Her research in this field includes the estimation of bone strength parameters using MATLAB and quantitative computed tomography analysis using MIMICS.
Learning Objectives
- Understand the challenges associated with mechanical testing for medical devices
- Learn about non-standard mechanical testing approaches to obtain material properties that can be used in FEA
Registration
The August 15, 2024 Customizing Mechanical Test Methods for Medical Device R&D web-based course is free, but registration is required and class size is limited.
Use our on-line registration form (see below) or print out this page and submit by one of the following:
- Scan and email to: seminars@veryst.com
- Fax to: 781.433.0933
- Mail to:
- Seminars
- Veryst Engineering
- 47A Kearney Road
- Needham, MA 02492
Deadline for registration is: Friday, August 9, 2024.
Cancellation Policy
Veryst reserves the right to reject registrations and to cancel a webinar based on class size.
Important Information
* You will receive an email confirmation once you have completed your registration.
* You will receive an email with login information the day before the webinar.