Technical Challenge
Batteries for electric vehicles are subjected to a variety of tests to characterize their performance and lifetime behavior. In the hybrid pulse power characterization (HPPC) test, a battery is subjected to sequential discharge and charge pulses over the range of the battery’s usable capacity and operating temperature. The voltage response of the battery during an HPPC test can be used to discern voltage losses due to (1) ohmic resistances, (2) kinetic activation of electrochemical reactions, and (3) concentration-dependent mass transport of charge carriers.
Veryst Solution
Veryst used COMSOL Multiphysics to simulate an HPPC test of a lithium-ion battery cell over a range of operating temperatures and initial states of charge. We used these simulations to determine the relative contributions of ohmic losses, activation polarization, and concentration polarization during battery operation.
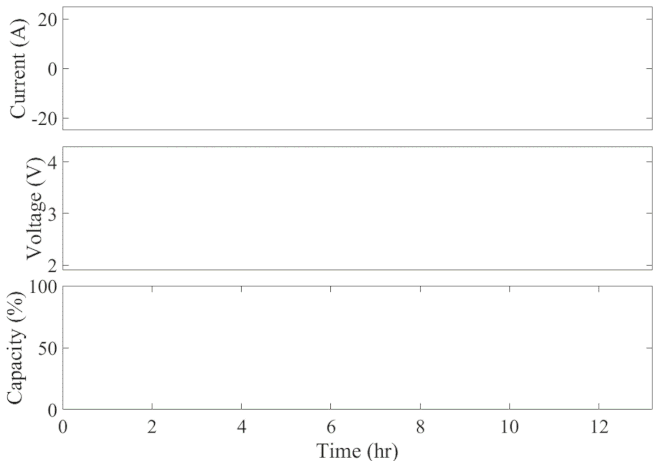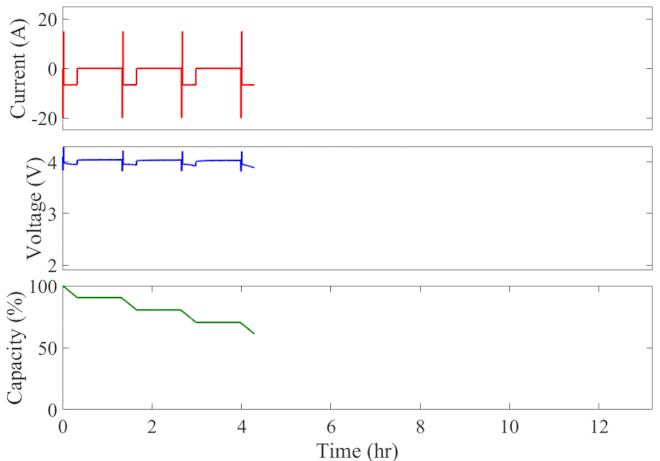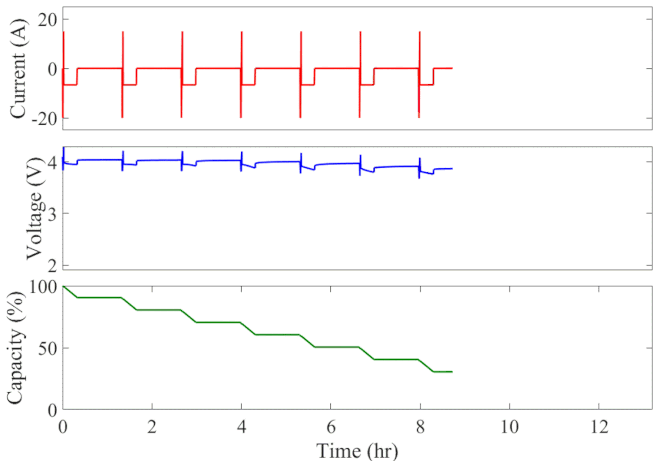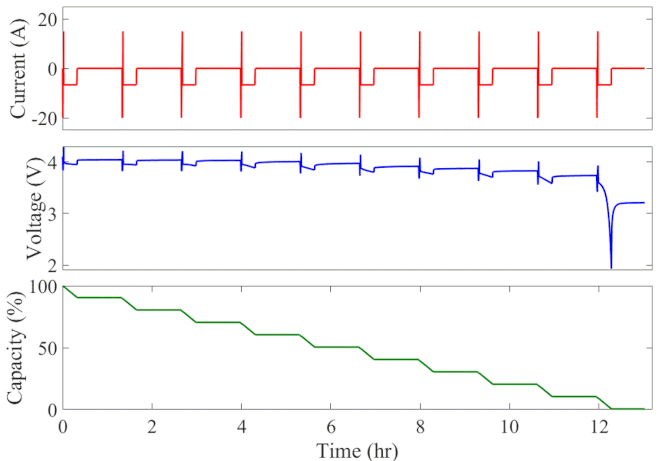
Well-calibrated simulations can expedite battery development by predicting performance under cyclic loading over long durations. Figure 1 animates the simulated current, voltage response, and state of charge of an initially fully charged battery over the course of the HPPC test. A short discharge-charge pulse is followed by a longer discharge period in which the battery is drained by 10% of its overall capacity. This cycle is repeated ten times, separated by a one-hour rest period, until the battery is completely discharged. The full test takes 13 hours to complete at a given temperature.
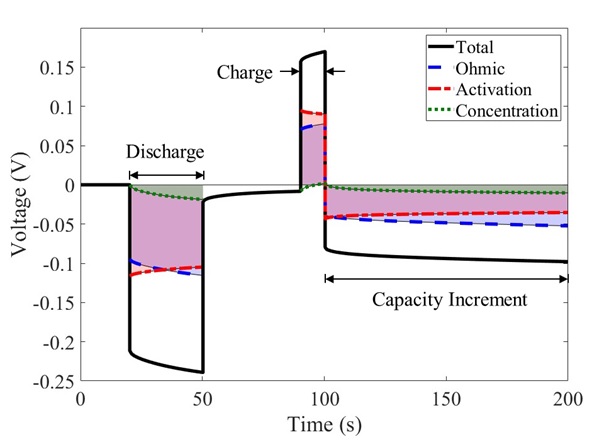
Each sequence of charge-discharge pulses reveals the competitive processes of ohmic resistance, activated electrode kinetics, and concentration-dependent mass transfer in the battery. Figure 2 plots the simulated voltage response from one cycle of the HPPC test, highlighting periods of discharge (30 seconds), charge (10 seconds), and capacity increment (18 minutes). Simulation allows direct computation of the voltage losses due to ohmic, activation, and concentration effects, also plotted versus time. Such information is not so easily measured experimentally.
Mass transfer is fast, indicated by the relatively small concentration polarization in Figure 2. The majority of the voltage response is attributed to a combination of ohmic and activation polarization. Activation losses initially dominate over ohmic losses following a step impulse. After a long hold period under constant current, the relative contribution of activation polarization lessens and the voltage response is dominated by the ohmic resistance in the cell.
To determine the relative contributions to voltage loss, we ran HPPC simulations across a range of operating temperatures and initial states of charge. We calculated the ohmic, activation, and concentration losses for the discharge and charge periods (Figure 3). Most of the cell polarization is attributed to ohmic and activation losses. The differential contributions between the discharge and charge pulses may be attributed to the shorter time of the charge pulse and charge relaxation following the discharge pulse.
Conclusion
Veryst performed COMSOL Multiphysics simulations of a lithium-ion battery cell undergoing an HPPC test. Our simulations showed that cell polarization is mainly attributed to activation and ohmic losses, with negligible effect of concentration-dependent mass transfer. We used these simulations to evaluate the battery performance across a wide range of operating conditions that would otherwise require days of experimental time.
Simulations such as these can be used to simulate battery cells and packs under a variety of tests, thereby reducing experimental time and cost and providing access to variables not easily measured.